A new study by researchers at Dartmouth’s Geisel School of Medicine sheds new light on a previously little understood area of fungal biology and could lead to new treatments for dangerous fungal pathogens.
The study published in the journal PLOS ONE, investigates the mechanisms of how HCR, a small segment of DNA, couples gene expression to morphology and describes the presence of HWP1 mRNA isoforms, which may be important for morphology specific expression.
“Morphology associated cell surface changes are notable for helping fungi to defend against immune attack, for establishing biofilm formation and growth in the host,” says Paula Sundstrom, PhD, a professor of microbiology and immunology at the Geisel School of Medicine. “Interference with the ability to couple gene expression to morphology will potentially lead to strategies for inhibiting fungal pathogen survival in host tissues—I hope our studies to understand how fungi couple gene expression to morphology will contribute to the battle against fungal diseases.”
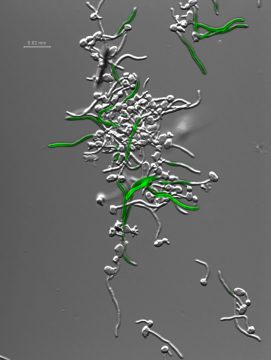
Many fungi are quick change artists, replacing one growth form with another while altering their surface coat and cellular metabolism—the triggers for these metamorphoses are environmental conditions that activate signal transduction pathways, affecting changes in gene expression profiles that optimize growth, Sundstrom explains.
Understanding the mechanisms that fungi use to co-regulate subsets of genes and morphogenesis is an unsolved problem in fungal biology and of keen interest to Sundstrom.
Candida albicans, a human fungal pathogen that is estimated to cause more than 400,000 life-threatening infections per year world-wide, rapidly undergoes interconversion between growth in budding yeast form and growth in the thread like form termed hyphae as part of its commensal and pathogenic lifestyles. To study how C. albicans couples changes in morphology to changes in gene expression, her laboratory studies the HWP1 gene, which is downstream of the HCR regulatory region. HWP1 encodes a surface protein that is specific to the hyphal growth form.
“Our results showed that in order for us to understand how fungal gene expression is coupled to morphology, we need to study mRNA isoforms associated with genes like HWP1 and ALS3 and not just the protein encoding genes themselves,” Sundstrom says.
Another finding is the discovery that HCR is required for universal expression of HWP1 on surfaces of all cells in a population—in strains lacking HCR, HWP1 is expressed on a fraction of cells during emergence of hyphal growth, showing that coupling of HWP1 to hyphal growth forms involves both HCR dependent and HCR independent mechanisms.
Support for this research was provided by the National Institute of Allergy and Infectious Diseases (R01 AI46608) and by the Geisel School of Medicine.