The BioRad ZE5 was formerly known as the Propel Labs YETI.


The ZE5 Cell Analyzer is a 27-color flow cytometer. For more information on the capabilities of the ZE5, click here. There are two ZE5 Cell Analyzers in the DartLab. Please contact DartLab staff to arrange for training if you would like to begin using these instruments.
Data Acquisition Software
Intuitive Everest software provides automated fluorescence compensation, a fluorochrome selector panel, and a runlist design wizard. Use this software on your computer to pre-configure the instrument settings, staining panels, and samples. Integrated training modules, and the ability to analyze files while acquiring saves time and streamlines your workflow. Everest software is PC-specific and is available at no charge from DartLab.
Optical Bench
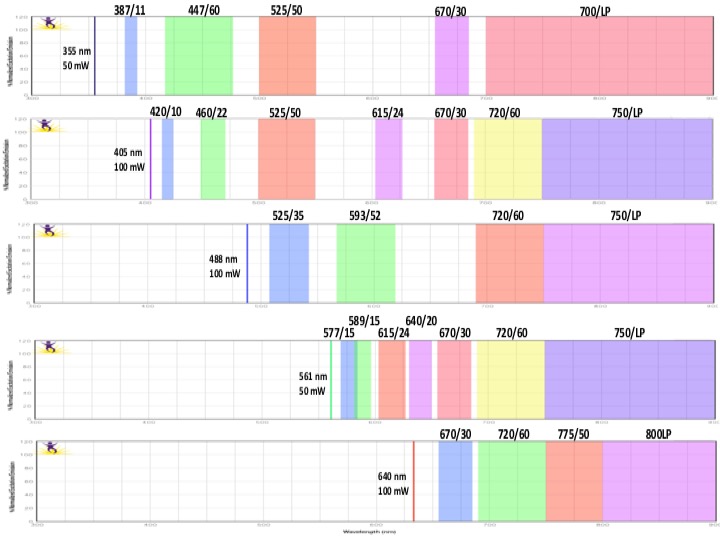
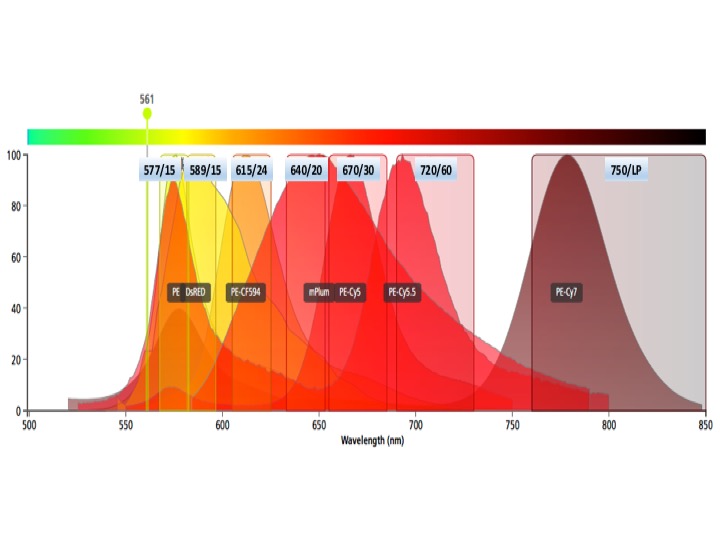
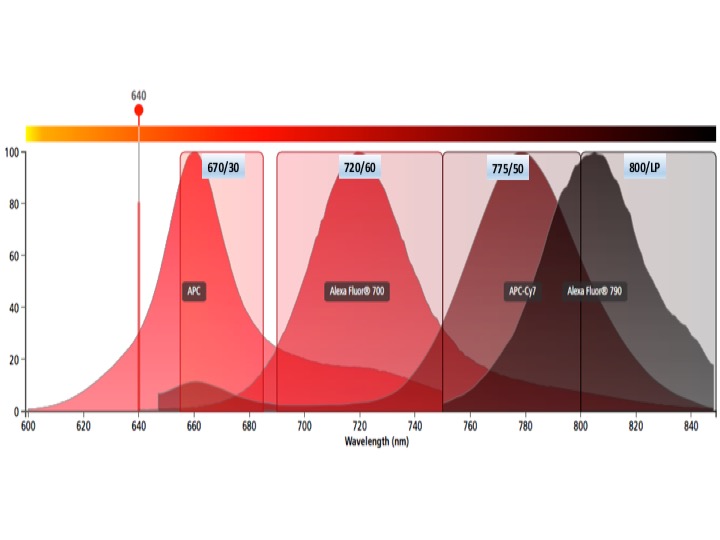
Each graph depicts a single laser and shows the band width for each detector. Be aware of inter-laser spectral overlap in detectors with similar band widths.
We have designed this template to download and enter fluors when designing a staining panel:

Data Analysis
"The high-throughput nature of flow cytometry, combined with the increasing capacity to measure more cell parameters at once, is generating massive and high-dimensional datasets on a routine daily basis. These data can no longer be adequately analysed using the classical, mostly manual, analysis techniques and therefore require the development of novel computational techniques, as well as their adoption by the broad community. Computational flow cytometry is a new discipline that straddles the fields of immunology and computational biology and provides a set of tools to analyze, visualize and interpret large amounts of cell data in a more automated and unbiased way." Saeys et al., 2016.
We recommend FlowJo V.10 for high-dimmensional data analysis. Please consult with us for guidance on data analysis strategies and use of plugins.
Available Fluorophores
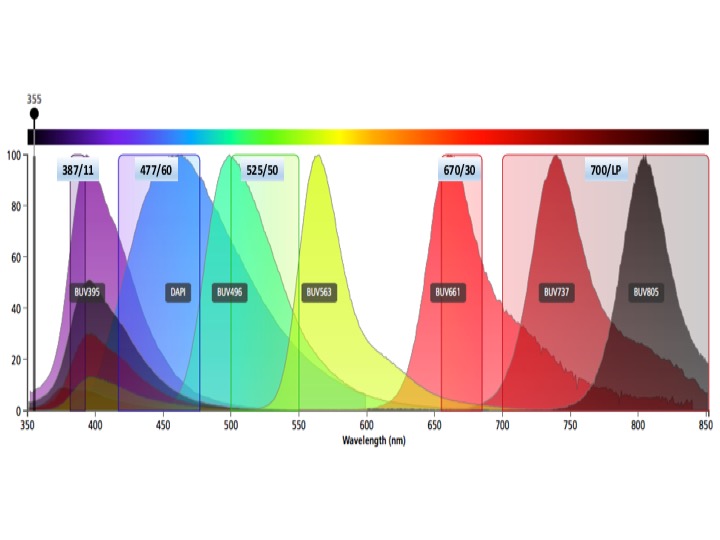
UV Laser Fluors
BUV395
AlexaFluor350 (AF350), DAPI, Hoechst-Blue, Indo-hi
BUV496, Indo-lo
BUV661
BUV737, BUV 805, Hoechst-red
Do not use BUV563
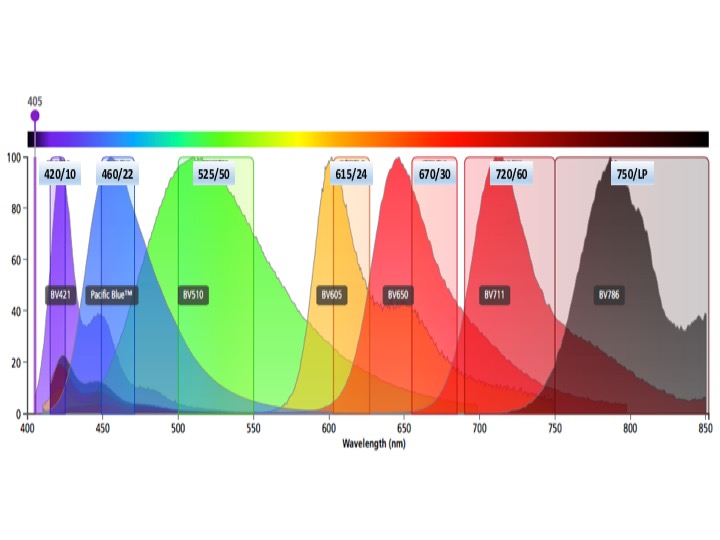
Violet laser fluors
BV421, Cascade Blue
BV450, Pacific Blue
BV510, AMCyan, Pacific Orange, Cascade Yellow
BV605
BV650
BV711
BV786
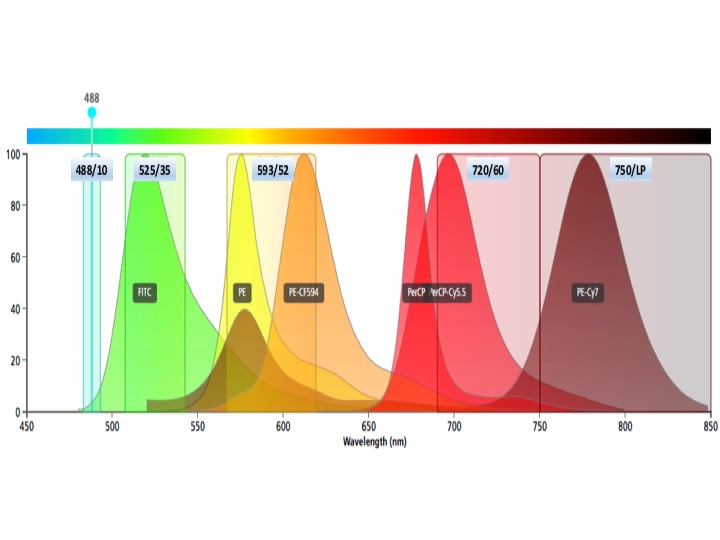
Blue laser fluors
Brilliant Blue 515, FITC, AF488, eGFP, eYFP
PE, PE-Dazzle, PE-CF594, PE-Texas Red (more useful off yellow laser)
PerCP-Cy5.5 but do not use PerCP (this is an excellent dump PMT)
PE-Cy7 (more useful off yellow laser)
Yellow laser fluors
PE
dTomato, DsRed
PE-Dazzle, PE-CF594, PE-Texas red
mPlum
PE-Cy5, PE-AF647
PE-Cy5.5
PE-Cy7
Red laser fluors
APC, AF647, Cy5
APC-R700, AF680, AF700, DRAQ5, Cy5.5
APC-Fire750, APC-Cy7, APC-H7, AF750
AF790
References
Start here with this excellent slide deck by Jonathan Irish.
Methods for discovery and characterization of cell subsets in high dimensional mass cytometry data (Diggins et al., 2015)
Characterizing cell subsets using marker enrichment modeling (Diggins et al., 2017)
A beginner's guide to analyzing and visualizing mass cytometry data (Kimball et al., 2018)