Pulmonary Immune Response to Aspergillus fumigatus
Typically, the respiratory immune system clears hundreds of Aspergillus conidia daily, but in immunocompromised individuals Aspergillus conidia can germinate in the lungs leading to the development of invasive aspergillosis. Currently, our knowledge of how Aspergillus fumigatus germination and growth is controlled in the respiratory tract is limited. Phagocytic alveolar macrophages and airway epithelial cells constitute the first lines of defense against inhaled A. fumigatus conidia; subsequently, neutrophils and macrophages are sequentially recruited to the respiratory tract to control fungal growth and germination. But how neutrophils and macrophages are recruited to and activated in the respiratory tract after inhalation of A. fumigatus conidia remains ill defined. Early after instillation of A. fumigatus conidia expression of leukotrienes and IL-1 cytokine family members is induced in the lungs.
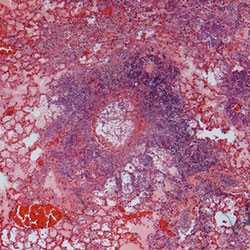
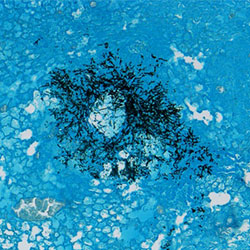 Lung histology stained with H&E (left) and GMS (right) from Kenalog immunosuppressed mice which were subsequently challenged with Aspergillus fumigatus (CEA10)
Lung histology stained with H&E (left) and GMS (right) from Kenalog immunosuppressed mice which were subsequently challenged with Aspergillus fumigatus (CEA10)
Our laboratory is currently using gene knockout mice, adoptive cell transfer, bone marrow chimeras, and therapeutic administration of cytokines and chemokines to explore the role of these inflammatory mediators in regulating immunity to A. fumigatus. These studies will expand our understanding of how A. fumigatus conidia are typically controlled in immunocompetent mammals. With this detailed understanding of the sequel of events necessary for leukocyte recruitment and activation to prevent invasive aspergillosis we can better understand how steroid or chemotherapeutic treatment alters anti-Aspergillus immunity to allow fungal growth and disease and also therapeutically manipulate these pathways to benefit patients with invasive aspergillosis.
