Our research aims to identify how innate immune pathways drive the development of autoimmunity and contribute to disease pathogenesis. We are addressing these questions in models of skin sterile inflammation that are relevant to autoimmune diseases such as systemic lupus erythematosus (SLE) and dermatomyositis (DM), as well as in primary human tissues. With the goal to identify novel therapeutic targets, we are investigating the regulation of type I interferon response as well as neutrophil migration and function.
Regulation of the type I interferon (IFN-I) response
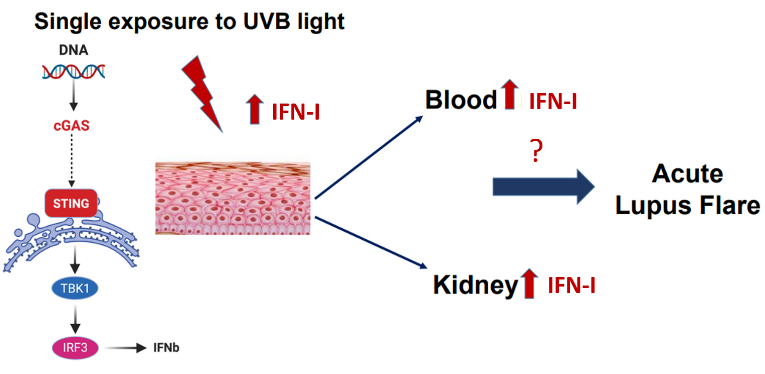 Sensitivity to UV light affects the majority of SLE and DM patients. The pathways predisposing these patients to We showed that a single exposure to UV light leads to a rapid IFN-I response in human and murine skin in vivo. This response was at least partially dependent on the DNA-sensing cGAS-STING pathway and not limited to the skin, as we detected high IFN-I signature in the blood and the kidney after UV exposure. That UV light is a potent stimulus of the IFN-I response is particularly relevant, since SLE and DM patients have a high IFN-I signature in both the skin tissue and the peripheral blood cells, indicative of worse disease. We aim to understand how the IFN-I production is (dys)regulated in SLE and DM skin and how this response can be modulated, both to suppress the high IFN-I signature in (a)symptomatic disease and to prevent UV-light triggered IFN-I production.
Sensitivity to UV light affects the majority of SLE and DM patients. The pathways predisposing these patients to We showed that a single exposure to UV light leads to a rapid IFN-I response in human and murine skin in vivo. This response was at least partially dependent on the DNA-sensing cGAS-STING pathway and not limited to the skin, as we detected high IFN-I signature in the blood and the kidney after UV exposure. That UV light is a potent stimulus of the IFN-I response is particularly relevant, since SLE and DM patients have a high IFN-I signature in both the skin tissue and the peripheral blood cells, indicative of worse disease. We aim to understand how the IFN-I production is (dys)regulated in SLE and DM skin and how this response can be modulated, both to suppress the high IFN-I signature in (a)symptomatic disease and to prevent UV-light triggered IFN-I production.
Neutrophil mechanisms of kidney injury in response to skin inflammation
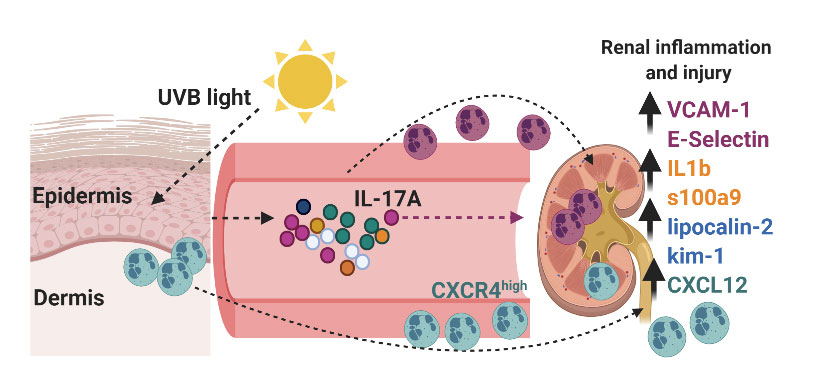 Can a walk in the sun cause kidney injury? For a large proportion of SLE patients, exposure to ultraviolet (UV) sunlight rays can trigger systemic flares, including kidney disease (lupus nephritis, LN). The cellular and molecular mechanisms linking skin inflammation and kidney injury in SLE in response to UV light are not understood. We showed that skin exposure to UV light stimulates inflammatory processes in the kidney, including expression of endothelial adhesion molecules, inflammatory mediators, renal injury markers, and transient proteinuria. We also discovered that neutrophils migrated to the kidney after skin UV exposure, localizing to the tubulointerstitial (TI) areas, where they exhibited a pro-inflammatory phenotype. Inhibiting neutrophil influx into the kidney abrogated renal inflammation and prevented upregulation of tubular injury markers. While our data suggest an important role for neutrophils, the mechanisms regulating neutrophil recruitment to the kidney and their contribution to kidney injury in response to UV are unknown. Therefore, there is a critical need to define how neutrophils traffic to and interact with renal tubular endothelium, especially since TI damage is predictive of LN. Moreover, we will investigate how these mechanisms influence disease under autoimmune conditions.
Can a walk in the sun cause kidney injury? For a large proportion of SLE patients, exposure to ultraviolet (UV) sunlight rays can trigger systemic flares, including kidney disease (lupus nephritis, LN). The cellular and molecular mechanisms linking skin inflammation and kidney injury in SLE in response to UV light are not understood. We showed that skin exposure to UV light stimulates inflammatory processes in the kidney, including expression of endothelial adhesion molecules, inflammatory mediators, renal injury markers, and transient proteinuria. We also discovered that neutrophils migrated to the kidney after skin UV exposure, localizing to the tubulointerstitial (TI) areas, where they exhibited a pro-inflammatory phenotype. Inhibiting neutrophil influx into the kidney abrogated renal inflammation and prevented upregulation of tubular injury markers. While our data suggest an important role for neutrophils, the mechanisms regulating neutrophil recruitment to the kidney and their contribution to kidney injury in response to UV are unknown. Therefore, there is a critical need to define how neutrophils traffic to and interact with renal tubular endothelium, especially since TI damage is predictive of LN. Moreover, we will investigate how these mechanisms influence disease under autoimmune conditions.