Tissue resident memory T cells (TRM) combine the exquisite specificity of adaptive immunity, with rapid frontline antiviral functions conventionally executed by innate immune cells.
Our lab is interested in understanding the function and regulation of TRM in distinct tissue environments to be able to contextualize their role in cancer, infection and autoimmunity.
Our current focuses
TRM in the brain
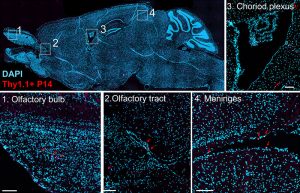
T cell functions in the central nervous system (CNS) are critical for protection against neurotropic pathogens, are implicated in a range of neurologic disorders, and even participate in normal CNS homeostasis. Despite this, there remain significant gaps in knowledge about how T cells, specifically TRM, are regulated and function in the imunologically unique brain environment. We are particularly interested in the role of inhibitory receptors such as PD-1 (which are elevated on brain TRM) in regulating responses. Using mouse models, we take a reductionist approach to study brain TRM to then be able to then understand the role these cells play in protective and pathologic settings such as vaccination, autoimmunity and cancer (see next project!).
TRM as a brain tumor immunotherapy
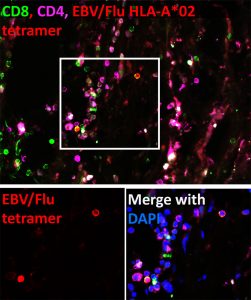
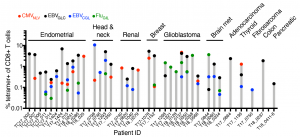
We found that tumors are populated by functional memory T cells specific for common viral infections (such as influenza, CMV and EBV). Remarkably, TRM with specificity for a single viral antigen can make up as much as 10% of the total CD8+ T cell population within a patient’s tumor. We can trick these cells into thinking a reinfection has occurred by delivering viral-derived peptides. This leads to TRM reactivation within the tumor and in mouse models of melanoma, immune activation and tumor regression.
Glioblastoma multiforme (GBM) is invariably fatal, notoriously immunosuppressive and novel therapies aimed at reversing the suppressive tumor environment are needed. Given this, we are interested in repurposing virus-specific TRM as a brain tumor immunotherapy.
TRM and oncolytic viral therapy
Oncolytic viruses (OV) are a promising class of cancer therapeutics that work by preferentially infecting and killing tumor cells. Currently there are over 100 clinical trials testing a range of oncolytic viruses and notably, the HSV-1 based OV, T-VEC, is used clinically for unresectable melanoma. Many OVs are engineered from viruses that the majority of the population has pre-existing T cell immunity to because of prior infection or vaccination (such as HSV-1, measles, and vaccinia virus), yet the impact of this immunity on patient outcome is unclear. We are interested in questions such as; what is the frequency of OV-specific TRM in tumors? Do these cells respond to OV therapy and what impact does this have on efficacy? Can we use this to refine and re-design OV therapies?