Research
In the Rigby lab, we conduct translational immunology research that encompasses different diseases, immunological processes, and therapeutic approaches. Our goal is to understand cellular and molecular events that occur in specific inflammatory settings, in hopes that this knowledge will lead to novel and improved therapeutic approaches in Rheumatoid Arthritis, Cystic Fibrosis, Chronic Lymphocytic Leukemia, etc. For these purposes, we have developed two clinical biorepositories for rheumatic diseases and knee osteoarthritis, which will allow us to better predict treatment outcomes including patient satisfaction. Brief description of the projects conducted in our lab can be found below.
Autoimmunity in Cystic Fibrosis
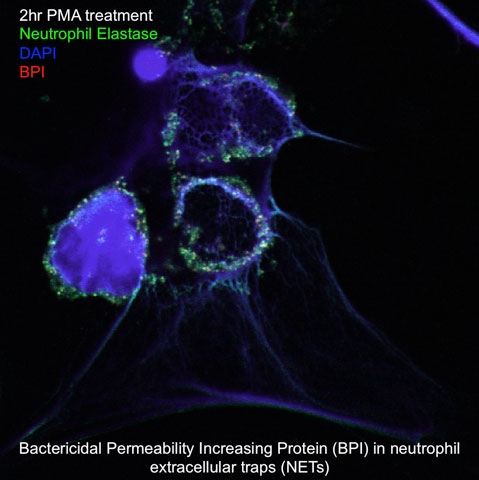 Cystic Fibrosis (CF) is an autosomal recessive genetic disorder that causes considerable morbidity and mortality. Chronic airway infection/inflammation with various pathogens, most notably Pseudomonas aeruginosa, is associated with this inflammatory process. While the CFTR mutation is associated with the defects in mucosal immunity, the relationship between microbiota and pulmonary function in CF patients is not well understood. Our project aims to understand the role of the adaptive immune response in the compromise of lung function in CF patients. Specifically, we are in interested in characterizing the development of autoimmunity to bactericidal-permeability-increasing (BPI) protein in CF patients, which has been associated with lung inflammation and injury.
Cystic Fibrosis (CF) is an autosomal recessive genetic disorder that causes considerable morbidity and mortality. Chronic airway infection/inflammation with various pathogens, most notably Pseudomonas aeruginosa, is associated with this inflammatory process. While the CFTR mutation is associated with the defects in mucosal immunity, the relationship between microbiota and pulmonary function in CF patients is not well understood. Our project aims to understand the role of the adaptive immune response in the compromise of lung function in CF patients. Specifically, we are in interested in characterizing the development of autoimmunity to bactericidal-permeability-increasing (BPI) protein in CF patients, which has been associated with lung inflammation and injury.
Novel biological activities of therapeutic monoclonal antibodies
 Therapeutic monoclonal antibodies rituximab (RTX) and obinutuzumab (GA101), directed against CD20 surface protein on B cells, have been widely used to treat B cell malignancies (e.g. chronic lymphocytic leukemia, CLL) and autoimmune diseases (e.g. rheumatoid arthritis, RA). Dr. Jones in our lab has discovered a novel biological activity mediated by these antibodies - trogocytosis, or "shaving". "Shaving" is a process where phagocytic cells, such as monocytes and PMNs, remove and endocytose RTX-bound CD20 molecule and surrounding proteins from B cell surface. This process alters both the phenotype and the genotype of the "shaved" B cell, which can impact the therapeutic efficacy of the monoclonal antibody. We are interested in understanding how trogocytosis contributes to the effector functions of anti-CD20 therapeutic monoclonal antibodies in different subsets of CLL patients. Moreover, we are investigating the impact of shaving on gene expression and function of B cells.
Therapeutic monoclonal antibodies rituximab (RTX) and obinutuzumab (GA101), directed against CD20 surface protein on B cells, have been widely used to treat B cell malignancies (e.g. chronic lymphocytic leukemia, CLL) and autoimmune diseases (e.g. rheumatoid arthritis, RA). Dr. Jones in our lab has discovered a novel biological activity mediated by these antibodies - trogocytosis, or "shaving". "Shaving" is a process where phagocytic cells, such as monocytes and PMNs, remove and endocytose RTX-bound CD20 molecule and surrounding proteins from B cell surface. This process alters both the phenotype and the genotype of the "shaved" B cell, which can impact the therapeutic efficacy of the monoclonal antibody. We are interested in understanding how trogocytosis contributes to the effector functions of anti-CD20 therapeutic monoclonal antibodies in different subsets of CLL patients. Moreover, we are investigating the impact of shaving on gene expression and function of B cells.
Total Knee Replacement Biorepository
While 75-80% of patients undergoing total knee replacement (TKR) surgery report improved pain and functional status, approximately 20% describe residual pain and instability. The ability to predict patient outcomes, such as the likelihood of rapid functional recovery following TKR, would both improve health care delivery as well as enhance the patients' ability to make informed decisions. In order to address this problem, we have initiated a biorepository of Synovium, plasma, RNA, and DNA from a highly characterized patient population being referred for TKR due to osteoarthritis. We are using the acquired biological material to identify and understand biomarkers that have the potential to increase success rates of total knee replacement.
Complement C4 Gene Copy Number Variation and Rheumatoid Arthritis
Rheumatoid Arthritis (RA) is a systemic inflammatory disease that localizes to the synovium of affected joints. It is strongly associated with Rheumatoid Factor (RF) or autoantibodies against citrullinated proteins (ACPA). ACPA positive RA patients commonly have the 'shared epitope', a 5 amino acid sequence [(R/Q)K(R)RAA] at position 70-74 in the HLA-DR beta (HLA-DRB) chain. Despite these advances, little is known about how the breaking of tolerance (evidenced by ACPA formation) leads to joint inflammation. Moreover, there are no predictive biomarkers of treatment response, resulting in empiricism and inefficiency. Given the relationship of ACPA and RF, most models suggest that RA results from immune complex formation either in the vasculature followed by synovial deposition or in situ in the joint. Immune complex handling is one of the major roles of the proximal components (C1, C4, C2) of the classical complement pathway. We propose that altered production of complement C4 either in the vasculature or in the joint play a key role in the induction of RA. In this regard, we have shown the genetic deficiency of complement C4 has been strongly associated (OR~3-5) with established RA in two completed studies, even with matching on the shared epitope or HLA-DRB1 alleles.