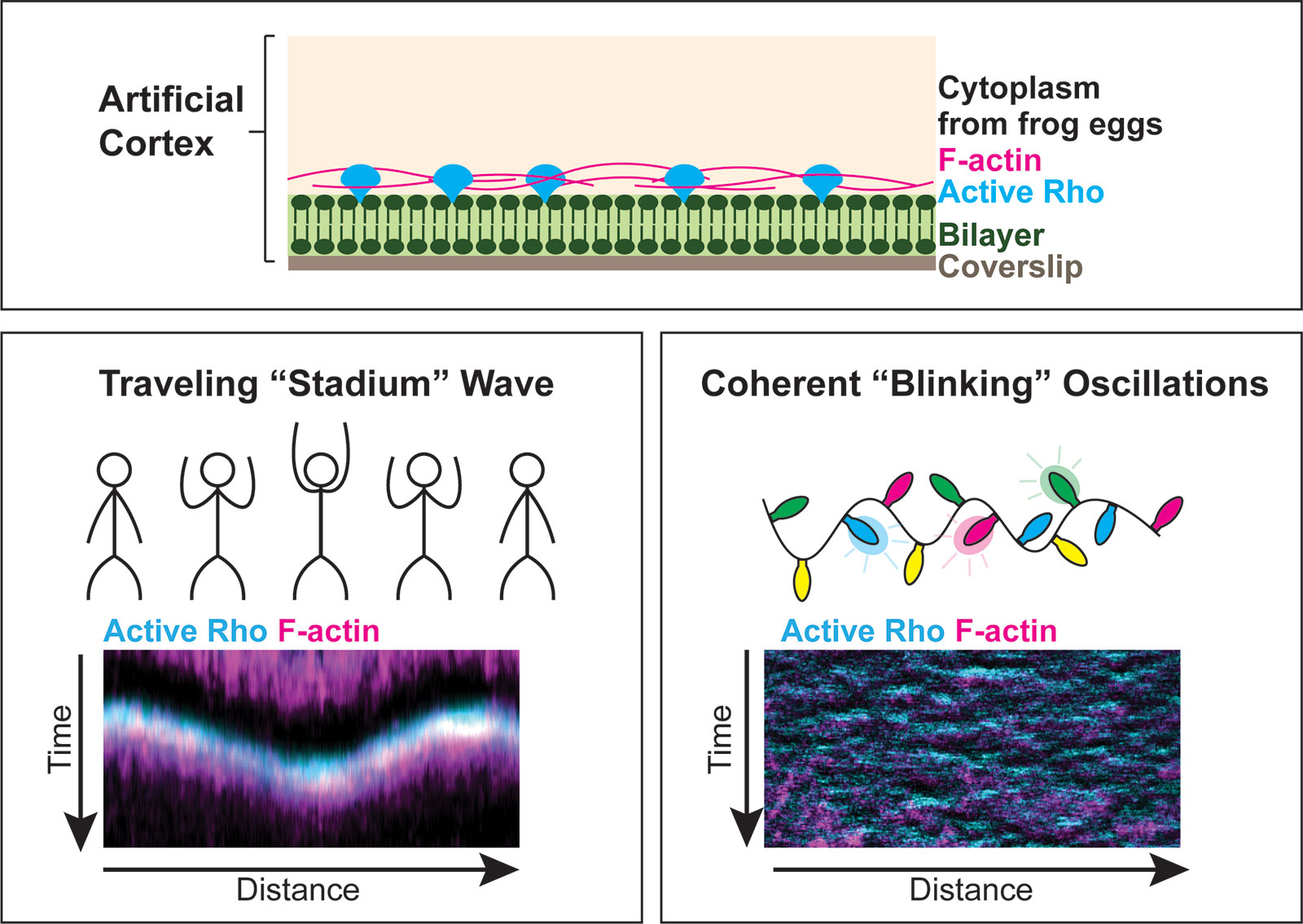
 Our lab is interested in how cells successfully divide into two daughter cells. This process ensures accurate segregation of the genome and cellular contents and is essential human development and disease. One outstanding question in the field is how the cell cortex is remodeled to support large-scale shape change during cytokinesis, the final stage of the cell cycle. It has recently been shown that the cell cortex is dynamically patterned prior to the onset of cytokinesis, and we are interested in defining the mechanisms of dynamic pattern formation at the cell cortex. This cortical patterning is self-organized and is driven by coupled positive and negative feedback signaling. We use synthetic approaches, including cell-free reconstitution of an artificial cell cortex, to study individual factors that support pattern formation like protein regulators, membrane composition and fluidity, and cell cycle state. The artificial cortex is an ideal system to answer such questions as it allows for direct manipulation of factors that are predicted to regulate cortical dynamics. Ongoing projects in the lab include the following:
Our lab is interested in how cells successfully divide into two daughter cells. This process ensures accurate segregation of the genome and cellular contents and is essential human development and disease. One outstanding question in the field is how the cell cortex is remodeled to support large-scale shape change during cytokinesis, the final stage of the cell cycle. It has recently been shown that the cell cortex is dynamically patterned prior to the onset of cytokinesis, and we are interested in defining the mechanisms of dynamic pattern formation at the cell cortex. This cortical patterning is self-organized and is driven by coupled positive and negative feedback signaling. We use synthetic approaches, including cell-free reconstitution of an artificial cell cortex, to study individual factors that support pattern formation like protein regulators, membrane composition and fluidity, and cell cycle state. The artificial cortex is an ideal system to answer such questions as it allows for direct manipulation of factors that are predicted to regulate cortical dynamics. Ongoing projects in the lab include the following:
1. Define the mechanism of cortical pattern initiation
Using the artificial cortex, we can observe the formation of excitable patterns from discrete foci. Additionally, we observe that supported lipid bilayer composition and fluidity impacts the type of pattern formation. We will manipulate upstream regulators of cortical patterning and investigate lipid composition to determine which factors predict the initiation of dynamic patterning.
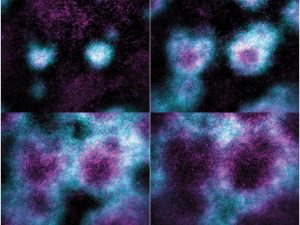
2. Investigate the organization of the cortical cytoskeleton
Multiple GTPases regulate the dynamics of the cytoskeleton at the cell cortex. We will test the role of additional GTPases in regulating patterning of actin filament assembly in the artificial cortex. Additionally, we will investigate the ultrastructural organization of cortical actin filaments to understand how patterning organizes F-actin to support cell division.
3. Define the relationship between cortical patterning and cell division
Investigating dynamic cortical patterning in live dividing cells is challenging, as cytokinetic furrow formation causes the cell cortex to change orientation and position and division progresses. The artificial cortex can be used to generate a two-dimensional model of the cytokinetic furrow which enables more straightforward live imaging and analysis. Using this approach we will test the relationship between cell cycle state and self-organized pattern formation along and characterize cortical patterns in reconstituted cytokinetic zones.