Information for Scientists
Our Center's mission is to identify and address exposures in early life related to a number of diseases in children.
The overarching goal of the Children’s Environmental Health and Disease Prevention Research Center at Dartmouth is to fill critical gaps in our understanding of the role of drinking water and food borne exposure to arsenic and potentially other contaminants in child growth, development and immune response. The Center supports multidisciplinary teams and innovative new technologies that are essential for conducting our research, and comprises an interdisciplinary team of scientists committed to advancing knowledge through three interwoven studies and two integrative cores.
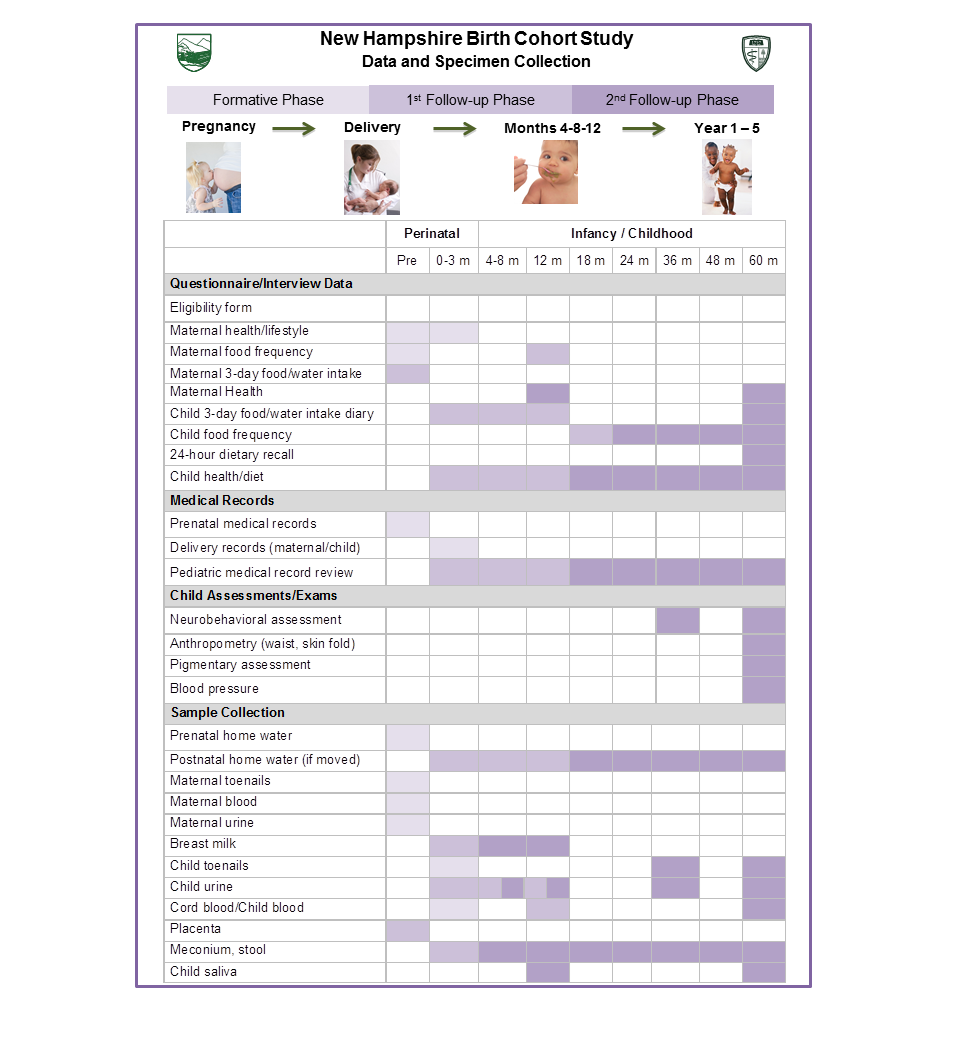
Project 1 builds on the ongoing New Hampshire Birth Cohort Study of pregnant women who use private wells in New Hampshire. Offspring of these women will be evaluated for occurrence of infections, allergies/atopy and vaccine response in the first years of life. Effects of arsenic on the developing microbiome also will be explored. The longitudinal birth cohort study will comprise about 1,500 mother-child dyads who are residents of New Hampshire and obtain household water from private wells, a potential source of arsenic exposure in the region. We actively follow infants enrolled in the cohort through interval interviews with the mothers every four to six months, and screen pediatric records up to age 5 years. We request a venous blood sample from infants during their one-year well-child visits to test for antibody response to diphtheria/tetanus/pertussis, pneumococcus and polio vaccines. We are sequencing bacterial DNA from repeated stool samples (collected at six weeks and 12 months of age) to assess the relation between in utero and early life exposure to arsenic on the developing microbiome.
Project 2 investigates the contribution of drinking water and diet to early childhood exposure to arsenic. By quantifying all routes of exposure, the impact of arsenic on children’s growth and neurodevelopment will be assessed. To characterize early childhood arsenic intake, we combine consumption data from repeated dietary assessments with arsenic concentrations measured in each child’s drinking water and foods. We then explore how intake through water and diet are associated with urinary and toenail biomarkers of arsenic exposure. These arsenic biomarkers then are evaluated for their association with altered growth (weight, length/height), and adiposity (body mass index, waist circumference, skinfold thicknesses) during the first five years of life, as well as impairment in behavioral skills and executive function at ages 3 and 5 years, and decrements in cognitive ability and motor proficiency at age 5 years.
Project 3 uses advanced techniques to examine epigenetic and gene expression alterations in the placenta in relation to arsenic exposure and health outcomes. We are focusing on the infant’s placenta as a relevant and functional tissue important in orchestrating fetal development and controlling the intrauterine environment. We are utilizing the tissues and data available in the NH cohort and are employing robust, sensitive assessments of gene expression and of genome-wide DNA methylation. These examinations allow us to understand potential mechanisms being altered with arsenic exposure and the consequences of their alteration on newborn and childhood health through the first 5 years of life.
We would like to thank our sponsors at NIEHS (P01ES022832) and the US EPA (RD83544201), as well as our New Hampshire Birth Cohort Study Participants.