Research
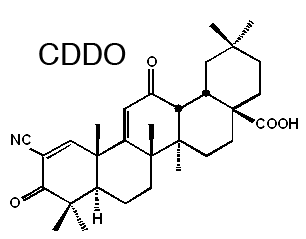
Synthetic triterpenoids are a promising new class of compounds with activity in a variety of preclinical cancer models. These multifunctional compounds are based on the natural product, oleanolic acid, as a starting material. Many of the new compounds that have been made, such as 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO), are highly potent (activities found at levels below 1 nM) anti-inflammatory agents, as measured by their ability to block the cellular synthesis of the enzyme inducible nitric oxide synthase (iNOS) in activated macrophages. Synthetic triterpenoids in in vivo studies include: 1) CDDO-Me, (bardoxolone methyl), the methyl ester of the synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO-Me); CDDO-Im, (CDDO-Imidazolide) 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole; and CDDO-EA (CDDO-Ethyl amide). CDDO-Me has been shown to be a potent chemopreventive agent in earlier studies. Sporn Laboratory studies have shown for the first time that the mammalian target of rapamycin (mTOR) is a direct target of CDDO-Im. We have also shown that CDDO-Im blocks insulin-induced activation of this pathway by binding to mTOR and inhibiting its kinase activity. Our basic studies confirm that CDDO-Im acts on a protein network to elicit its pharmacological activity.
Focus has been on four particular diseases in recent and ongoing studies: the prevention and treatment of breast cancer, prevention and treatment of lung cancer, prevention of pancreatic cancer, and the treatment of osteoarthritis.
For arthritis treatment, novel methods for inducing chondrogenesis are critical for cartilage tissue engineering and regeneration. In collaboration with Nanjoo Suh at Rutgers and Damian Medici at Harvard, Sporn Laboratory studies have shown the first report of synthetic oleanane triterpenoids, CDDO-Im and CDDO-EA to induce chondrogenesis in organ cultures of newborn mouse calvaria. A follow-up study of treatment of human bone marrow-derived mesenchymal stem cells with CDDO-Im and CDDO-EA showed induction of expression of SOX9, collagen IIα1, and aggrecan, as well as BMP-2 and phospho-Smad5, confirming that CDDO-Im and CDDO-EA induce chondrogenic differentiation.
The breast cancer-associated gene 1 (Brca1) is the most frequently mutated tumor suppressor gene in familial breast cancers. Mutations in Brca1 also predispose to other types of cancers, pointing to a fundamental role of this pathway in tumor suppression and emphasizing the need for effective chemoprevention in these high-risk patients. We tested CDDO-Me's efficacy in a highly relevant mouse model of Brca1-mutated breast cancer. CDDO-Me significantly delayed tumor development in Brca1-mutated mice. We also observed that levels of ErbB2, p-ErbB2, and cyclin D1 increased in a time-dependent manner in the mammary glands in Brca1-deficient mice, and CDDO-Me inhibited the constitutive phosphorylation of ErbB2 in tumor tissues from these mice. In Brca1-deficient cell lines, the triterpenoids directly interacted with ErbB2, decreased constitutive phosphorylation of ErbB2, inhibited proliferation, and induced G(0)-G(1) arrest. These results suggest that CDDO-Me has the potential to prevent BRCA1-mutated breast cancer.
We tested activity of CDDO-Me again in a relevant model of estrogen receptor-negative breast cancer, the polyoma-middle T (PyMT), in which the oncoprotein drives carcinogenesis. CDDO-Me significantly increased the age of mice at onset of first tumor, increased overall survival, inhibited the infiltration of tumor-associated macrophages into mammary glands of PyMT mice, and reduced levels of the chemokines CXCL12 and CCL2 in primary PyMT mammary tumor cells. Furthermore, treatment with this multifunctional drug also inhibited secretion of matrix metalloproteinase-9 in primary tumor cells from PyMT mice and decreased proliferation of these cells by inhibiting cyclin D1 and decreasing phosphorylation of epidermal growth factor receptor and signal transducer and activator of transcription 3 (STAT3).
Pancreatic cancer is the fourth leading cause of cancer-related deaths in the United States and is nearly always fatal. Whereas early detection offers the most promising approach for reducing the mortality of this disease, there is still a need to develop effective drugs for the prevention and treatment of pancreatic cancer. We tested two promising classes of non-cytotoxic drugs, synthetic oleanane triterpenoids and rexinoids, as single agents or in combination, for the prevention of carcinogenesis in the highly relevant KPC mouse model of pancreatic cancer. KPC transgenic mice closely recapitulate the genetic mutations, clinical symptoms, and histopathology found in human pancreatic cancer. CDDO-Me, LG268, the combination of CDDO-Me and LG268, and the combination of CDDO-ethyl amide and LG268, all significantly increased survival in the KPC mice. In contract, recent studies have shown that gemcitabine, the current standard of care for human pancreatic cancer, does not extend survival in KPC mice. In cell lines developed from the KPC mice, the triterpenoids directly interact with both STAT3 and IκB kinase (IKK) to decrease constitutive interleukin-6 secretion, inhibit constitutive STAT3 phosphorylation, and block the degradation of IκBα when challenged with tumor necrosis factor α. These results suggest that oleanane triterpenoids and rexinoids have the potential to prevent pancreatic cancer.
