Epigenetic Alterations in Cancer
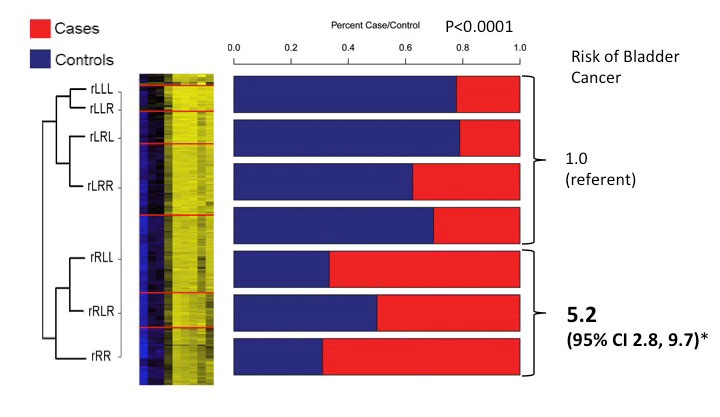
Human cancers are characterized by a an overt hypomethylation of their DNA, which likely effects highly repetitive and fragile regions of the genome, while paradoxically exhibiting hypermethylation of the promoter regions of tumor suppressor genes. While these characteristics are well described, their etiology and potential clinical utility remain significantly understudied. Work in our laboratory has focused on cancers with known environmental exposure etiologies, specifically, head and neck cancer, bladder cancer, lung cancer and malignant mesothelioma. The etiologies of these diseases are disparate, and include tobacco smoke, arsenic and asbestos exposures, and nutrition and dietary factors. In bladder cancer, our work has aimed to understand the relationship between environmental exposures important in this disease, including tobacco smoking and arsenic, and epigenetic alterations. This work was a collaborative effort between Dr. Karl Kelsey at Brown, Dr. Margaret Karagas at Dartmouth and myself, and has taken advantage of the population-based case-control study of bladder cancer in New Hampshire spearheaded by Dr. Karagas. Using these unique resources, we have demonstrated that both gene-specific DNA hypermethylation (Marsit Carcinogenesis 2006, Marsit Cancer Res 2005) as well as overall propensity for hypermethylation (Marsit Carcinogenesis 2007) are associated with these exposures including tobacco smoke and arsenic, and can serve as markers of tumor aggressiveness and patient survival. Building upon this research, we have now begun to examine how epigenetic alterations not only contribute to carcinogenesis in the target tissues, but also may serve as novel markers of susceptibility. We have demonstrated (Wilhelm et al, Clin Canc Res 2010) that alterations to the global levels of DNA methylation in peripheral blood cells are associated with arsenic exposure and act as a marker of bladder cancer risk, specifically in women. We have also utilized a novel array-based approach to identify profiles of gene-specific methylation which are associated with bladder cancer risk, and have developed risk prediction models based on these profiles which rival those using genetic susceptibility factors (Marsit et al J Clin Oncol 2011). Our most recent studies have aimed to better identify what these altered profiles represent (Langevin et al Epigenetics 2011) and to utilize novel statistical methodologies coupled with biological knowledge to improve their predictive capabilities (Koestler et al CEBP 2012; Houseman et al BMC Bioinformatics 2012).
Together, these approaches focused both on target tissue somatic alterations and on novel biomarkers of disease susceptibility are driving the future of my research program in human cancer specifically focused on bladder cancer. Beyond understanding the etiology of this disease, a major clinical question with clear translational appeal is identifying those factors that are associated with bladder cancer recurrence and progression. Although the mortality of bladder cancer is low, the morbidity is significant, as patients presenting with even early stage, non-invasive disease must be frequently monitored with painful, costly, invasive procedures in order to detect and treat disease recurrence and to monitor progression. Future work in the laboratory will examine how such biomarkers in accessible tissues, including urine and blood, can be used to build improved models of recurrence and progression, in collaboration with Dr. Schned in Pathology, and Dr. Seigne in Urology.