Neurons employ a variety of mechanism to regulate their intrinsic excitability. Several mechanisms are known to reduce excitability in proportion to action potential output, thereby acting as negative feedback to prevent excessive excitability. These mechanisms generate what are known as afterhyperpolarizations that generate a net outward current to hyperpolarize neurons and reduce their likelihood of firing action potentials. Cortical pyramidal neurons are known to have particularly long-lasting AHPs, and a variety of earlier work has implicated calcium- and/or sodium-dependent potassium channels as mediating this change in excitability. We have recently examined the ionic basis for these long-lasting AHPs in neocortical layer 5 and hippocampal CA1 pyramidal neurons using combined electrical and optical recording. Our results demonstrate that the majority of the AHP is sodium-, not calcium-, dependent, and is due to activation of the sodium-potassium ATPase (i.e., the "sodium pump"), rather than a potassium conductance.
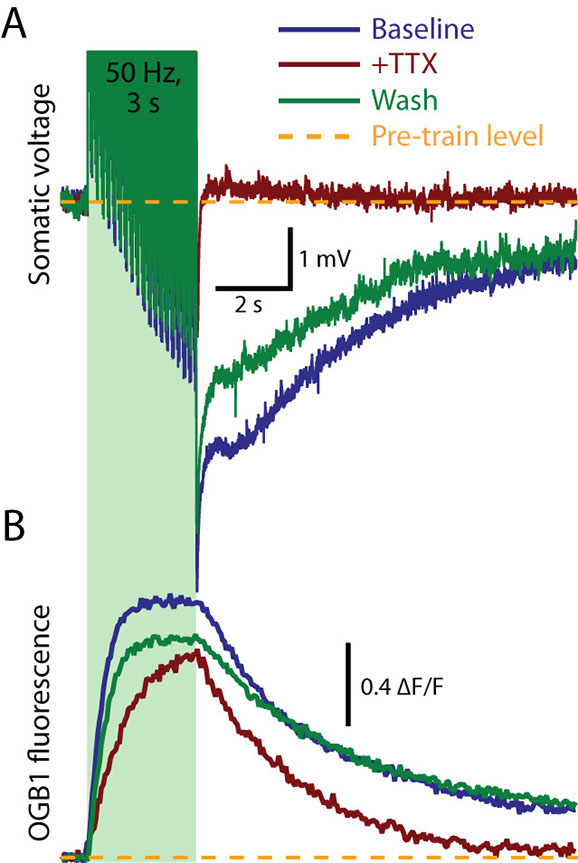
A sodium-dependent long-lasting AHP in layer 5 pyramidal neurons. A: Recordings of the membrane potential of a layer 5 pyramidal neuron in baseline conditions (blue), following focal application of TTX (red), and after several minutes of wash (green).Short (2 ms), high amplitdue (3 nA) current injections were used to generate 150 action potentials (or action-potential-like depolarizations) at 50 Hz (green column). Spikes are truncated for better visualization of the AHP that follows the spike train. Note that TTX reversibly blocks all but the initial moments of the AHP. BThese same neurons were filled with the calcium-sensitive dye Oregron Green BAPTA-1 (OGB1), allowing simultaneous optical detection of intracellular calcium accumulation during (and following) the spike train.The presence of TTX had only a small effect on peak intracellular calcium, suggesting that calcium may not be a primary mediator of the long-lasting AHP. (Adapted from Gulledge et al., . Journal of Neuroscience, 33:13025–13041, 2013).