Background
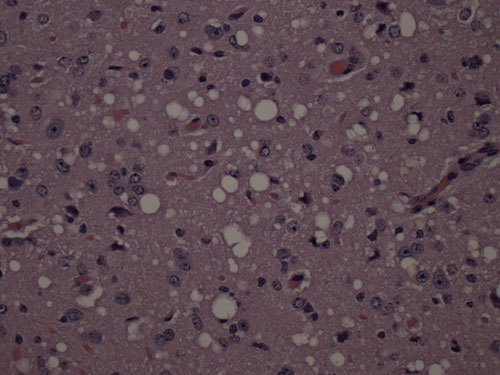
Prions are infectious agents of fatal brain diseases that include: Creutzfeldt Jakob Disease (CJD) and kuru in humans, bovine spongiform encephalopathy (BSE) in cows, Chronic Wasting Disease (CWD) in deer and elk, and scrapie in sheep and goats. Interestingly, prions are unorthodox infectious agents because they do not contain nucleic acids. Instead, a membrane-bound glycoprotein called the prion protein (PrP) appears to be the molecule responsible for the transmission of prion disease. The native, cellular conformer of PrP, designated PrPC, is expressed in all normal mammals. When an animal contracts prion disease, the PrPC molecules in the brain of that animal undergo a conformational change to a pathogenic conformer designated PrPSc. This pathogenic conformer is infectious, promoting conformational change to create more PrPSc molecules, and its formation leads to the death of neurons.
Interestingly, recent studies have shown that a number of other neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, Huntington's disease, and Amyotrophic Lateral Sclerosis (ALS) apparently also spread through the brain using prion-like mechanisms. Furthermore, prion-like proteins have also been found in non-mammalian organisms such as bacteria and yeast, where they can serve normal biological functions. Thus, studies of infectious prions are likely to have far-reaching impact in both medicine and biology.
Currently, the structure and composition of infectious prions, as well as the mechanisms by which these agents replicate and destroy neurons are unknown. These questions represent key areas of investigation for our laboratory. Significant scientific advances made in our laboratory include the de novo formation of infectious prions from defined, non-infectious components in vitro, the identification of RNA and phosphatidylethanolamine (PE) as endogenous cofactors in prion formation, and the demonstration that such cofactors encode infectivity and strain properties.