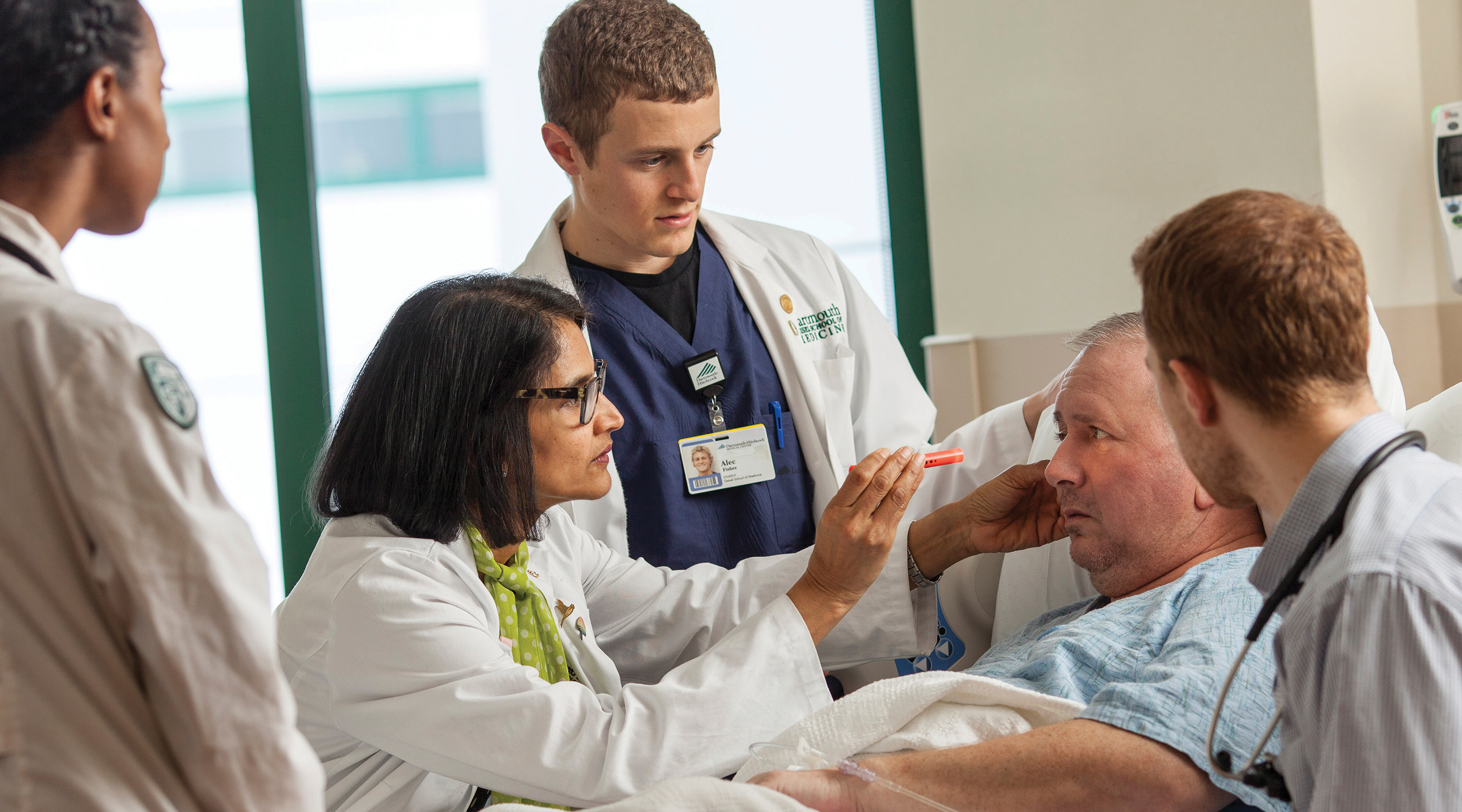
Dartmouth and its Geisel School of Medicineare forging that future. We invite you to join us in this call to lead.
Dartmouth, Dartmouth-Hitchcock Health Launch Major Cancer Research Institute
A $25 million gift to Dartmouth and Dartmouth-Hitchcock Health from Dorothy Byrne, a long-time supporter of cancer research and patient care, will establish a robust, interdisciplinary cancer research institute to leverage the innovation, entrepreneurship, and collaboration that is deeply embedded in the Norris Cotton Cancer Center (NCCC).
Campaign Goals
As a core part of The Call to Lead campaign, Geisel seeks $200M in new philanthropic support to advance the following areas:
$180 Million for a Healthy, Resilient, Equitable Future
More than 3,700 alumni, parents, and friends have answered Dartmouth’s ongoing “Call to Lead” campaign with gifts to the medical school, bringing it within reach of its $207-million goal. Click “Read More” to watch the video.
Diversity, Equity, and Inclusion
Learn how you can help advance diversity, equity, and inclusion at Geisel.