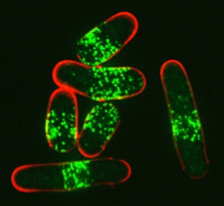
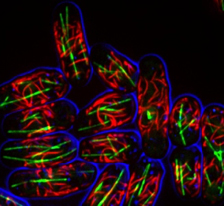
We study how cells coordinate cell growth and division. At the heart of this coordination are signaling pathways that link cell growth proteins with the core cell cycle machinery. We use a multi-disciplinary approach to identify these pathways, and then to understand how their activities are controlled by changes in cell size and shape. As many of these proteins are found at distinct sites in the plasma membrane, we have also become interested in the organizational principles that generate discrete compartments at the cell cortex. We use fission yeast cells as a model system because they allow us to combine a wide range of genetic, genomic, biochemical, and microscopy techniques. In addition, the basic cell growth and cell cycle systems are well conserved between fission yeast and human cells, where they have important links to the generation of cancer.
Ongoing projects in the lab include:
- How do cells stay the right size?
Many cells delay cell cycle transitions until they grow to a critical size threshold, but the mechanisms that measure size and transmit this information are largely unknown. Fission yeast cells grow to a reproducible size before entering into mitosis and dividing. We are studying a conserved signaling pathway that controls this decision to divide. The conserved components of this pathway assemble into “nodes,” which are megadalton structures at the plasma membrane. We have discovered upstream regulatory factors that control node activity according to cell size and growth. We have also uncovered how nodes communicate with the core cell cycle machinery. Ongoing efforts are focused on how organization into megadalton nodes helps this size-dependent pathway to operate reliably. - Cellular adaptation to environmental stress.
Cells encounter rapid changes in nutrients, temperature, osmolarity, and more. Mechanisms exist to help cells continue growth and division in a fluctuating environment. We have focused on a conserved family of protein kinases called AMPK-related kinases (ARKs) that play important roles in this process. Different ARKs are turned on and off at different times and places, and defects in their coordination are associated with human cancers. We have developed yeast as a simplified system to understand this conserved signaling system, and have identified an unexpected role for protein phosphatases in connecting ARKs with the cellular environment. - Novel cytoskeletal filaments at the cell cortex.
We have identified a protein that assembles into filaments both in vitro and in cells. These filaments are independent of known cytoskeletal elements, and thus represent a new cytoskeletal structure. We have used yeast genetics to determine how these filaments control conserved signaling pathways, and continue to investigate the underlying molecular mechanisms. In addition, we are examining the biochemical mechanism and structure of these filaments using combined approaches in vitro.