| Watch this award-winning video to learn about rotations and meet members of the lab! Filmed by Abby Dutton. |
|
Herpes simplex virus (HSV) is a highly evolved and successful pathogen with a very wide distribution in the human population, close to 100% in some populations. HSV often manages to forge the near-perfect lifelong relationship with us, its human host. Despite the availability of excellent antiviral drugs such as acyclovir and valtrex, HSV is refractory to cure. This is due to the ability of HSV to invade neurons, to translocate to neuronal cell bodies, and establish latent infections therein. The virus may subsequently reactivate (see movie narrated by a Cockney gangster) producing new infectious virus and spread to susceptible individuals in the population. Our over-arching interest is to understand how HSV modulates the immune system of the host. This immune-modulation allows HSV to successfully establish latency, and more remarkably, to allow the virus to escape from the immune host to infect other susceptible individuals in the population. Our experimental approach is to use forward and reverse genetics to introduce mutations into the viral genome to generate recombinant viruses. We then use these recombinant viruses in vitro and in vivo to allow the study of viral pathogenesis at the molecular level, and to examine the outcome of the host-pathogen battle. |
|
|
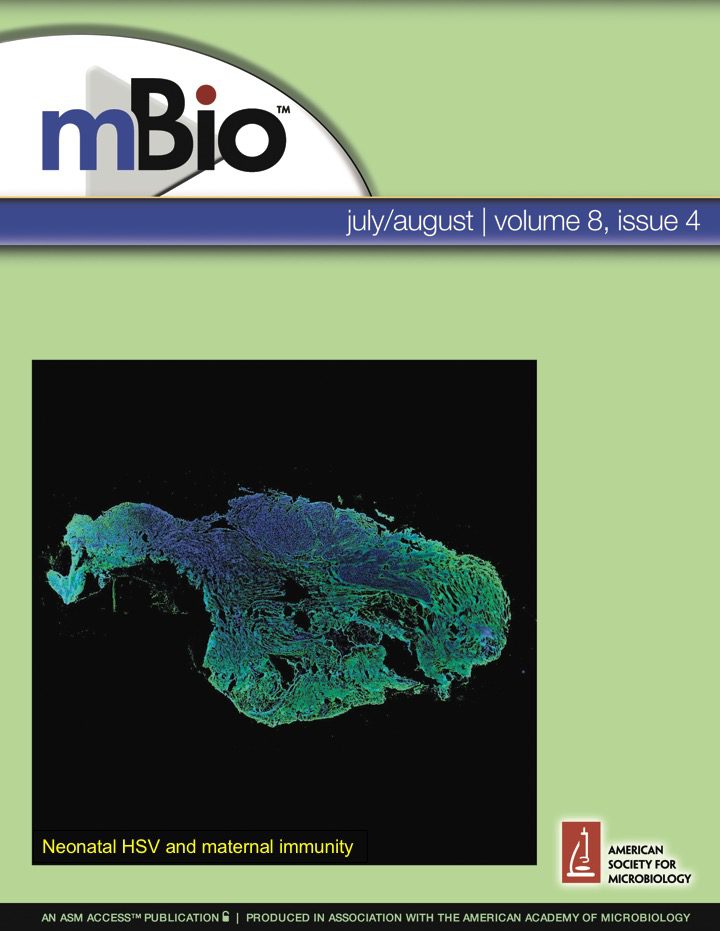
Herpes simplex virus neonatal infections and maternal immunity Herpes simplex virus type-1 is a common infection of the nervous system that causes devastating neonatal disease. Using mouse and human tissue, we discovered that antiviral antibodies accumulate in neural tissue after HSV-1 infection in adults. Similarly, these antibodies pass to the offspring during pregnancy. We have recently shown that antiviral maternal antibodies can readily access neural tissue of the fetus and neonate. These maternal antibodies then protect neonatal mice against HSV-1 neurological infection and death. Our work has shown the previously unappreciated role of maternal antibodies in protecting fetal and newborn nervous systems against infection. Recently, we've shown that maternal immunization is efficacious at preventing neonatal herpes infections. Ongoing work in the lab is testing new vaccines and antibody approaches against neonatal HSV disease, and probing the specificity and mechanisms of protection of these antibodies. |
|
| HSV and modulation of autophagy and innate immunity
Autophagy is a constitutive cellular process in which cytoplasmic components are sequestered and degraded by the lysosome to generate metabolic precursors, to remove damaged organelles and altered intracellular components. If the autophagic vacuole also engulfs and destroys invading pathogens, the process is known as xenophagy -- an arm of the intrinsic immune response. This is distinct from the type of innate defenses that are typified by the establishment of the IFN-driven antiviral state. Three important aspects of HSV and intrinsic and innate defense are being studied. First, we are determining the role of autophagy in control of HSV-1 infection in neurons. Second, we are exploring mechanisms by which viral genes, especially γ34.5, subvert the host xenophagy and interferon (IFN)-mediated antiviral responses. Finally we are investigating how the IFN responsiveness of the immune system determines viral tropism and damage during visceralHSV infections using real-time bioluminescence and adoptive immune transfer technologies.
|
Journal Cover Images from the Leib Lab: