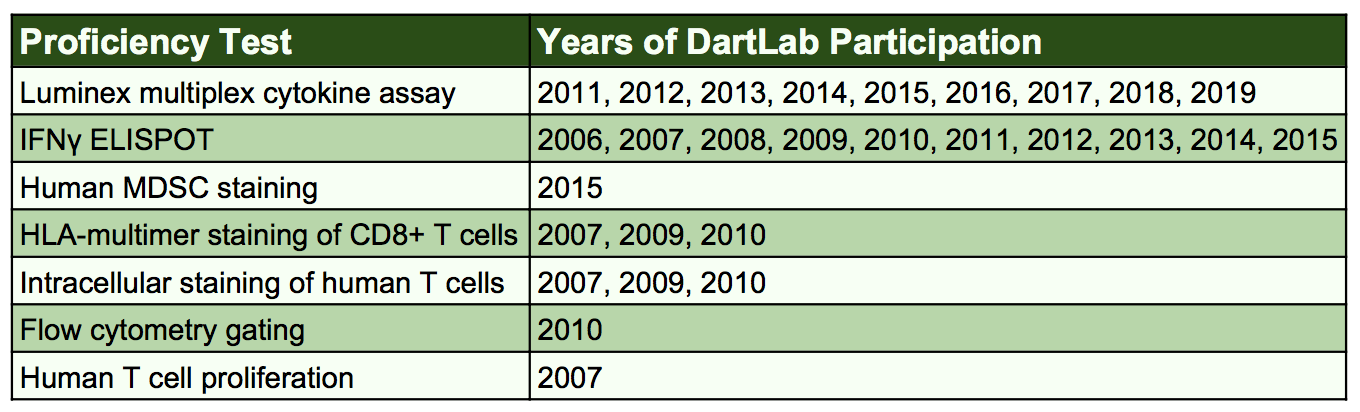
Proficiency testing is integral to the successful operation of a Shared Resource. The consistently high performance of all DartLab technologists on external proficiency test allows us to be confident that our results are accurate and precise. Proficiency testing demonstrates that our results are similar to those of multiple other laboratories, both nationally and internationally, when identical cells or analytes are assayed. DartLab technologists are well-trained and use defined standard operating procedures (SOPs), and calibrated and maintained instrumentation.
Assay Harmonization
The harmonization process involves the participation of multiple laboratories in a consortium-based, iterative testing process to identify the variables crucial for assay performance. Each participating laboratory uses its own reagents and instrumentation and performs quality control experiments on replicate samples. A central laboratory manages logistics for the proficiency panel, receives raw and analyzed data sets from each participating laboratory, and provides independent central data analysis.
During initial proficiency panels, variables that impact laboratory performance are identified. Subsequent panels harmonize use of performance-stabilizing variables across laboratories. The outcome of this iterative process is a set of harmonization guidelines that can be implemented both by the panel participants and by the larger scientific community. Harmonization can be applied to all assay variables from the protocol and raw data acquisition, processing, interpretation, and reporting, so that comparison of data between laboratories becomes objective and supportive of immune biomarker research (van der Burg et al., 2011).
The primary outcome of harmonization is that data sets generated across multiple harmonized laboratories are directly interpretable with regard to test performance. These independent and harmonized data sets from multiple laboratories have facilitated the establishment of assay-specific reference values for background, lower limits of detection, and replicate variation, which serve as community-wide benchmarks for assay performance (Moodie et al., 2012).
The results of proficiency testing have been published in important assay harmonization papers (see below), and have culminated in proposed Minimal Information About T cell Assays (MIATA) (Janetzki et al., 2009; www.miataproject.org) and MIFlowCyt: the minimum information about a Flow Cytometry Experiment (MIFlowCyt; Lee et al., 2008). These are minimum criteria for reporting immune-monitoring experiments and results in order to facilitate more objective data interpretation and meta-analysis.
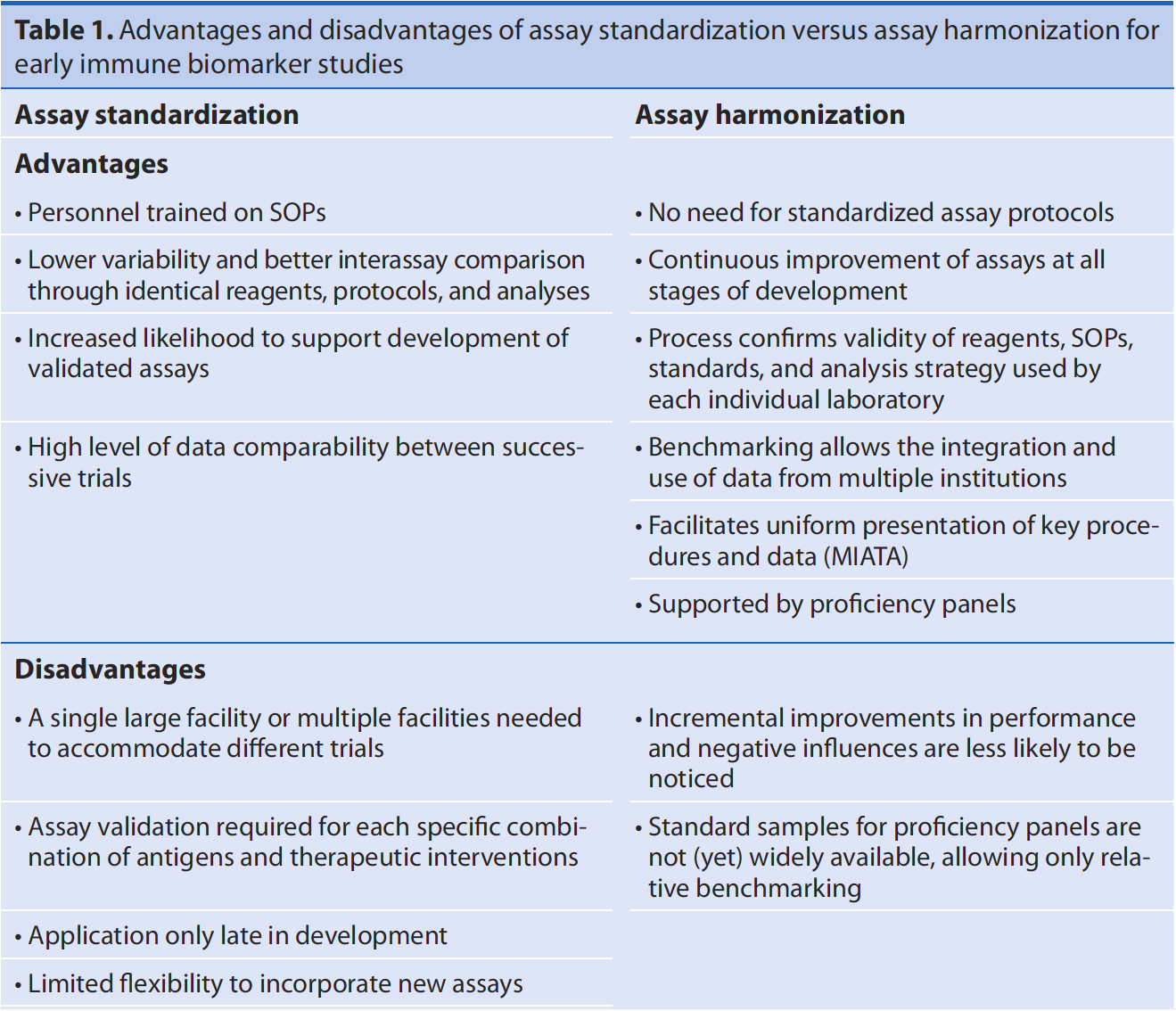 Table 1: Comparison of assay standardization versus assay harmonization from van der Burg et al. (2011).
Table 1: Comparison of assay standardization versus assay harmonization from van der Burg et al. (2011).
Janetzki S, Hoos A, Melief CJ, Odunsi K, Romero P, Britten CM. Structured reporting of T cell assay results. Cancer Immun. 2013;13:13. PubMed PMID: 23882158.
McNeil LK, Price L, Britten CM, Jaimes M, Maecker H, Odunsi K, Matsuzaki J,Staats JS, Thorpe J, Yuan J, Janetzki S. A harmonized approach to intracellular cytokine staining gating: Results from an international multiconsortia proficiency panel conducted by the Cancer Immunotherapy Consortium (CIC/CRI). Cytometry A. 2013;83:728-38. PubMed PMID: 23788464.
Janetzki S, Panageas KS, Ben-Porat L, Boyer J, Britten CM, Clay TM, Kalos M, Maecker HT, Romero P, Yuan J, Kast WM, Hoos A; Elispot Proficiency Panel of the CVC Immune Assay Working Group. Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI). Cancer Immunol Immunother. 2008;57:303-15. PubMed PMID: 17721781.
Janetzki S, Britten CM, Kalos M, Levitsky HI, Maecker HT, Melief CJ, Old LJ, Romero P, Hoos A, Davis MM. "MIATA"-minimal information about T cell assays. Immunity. 2009;31:527-8. PubMed PMID: 19833080.
Attig S, Price L, Janetzki S, Kalos M, Pride M, McNeil L, Clay T, Yuan J, Odunsi K, Hoos A, Romero P, Britten CM; CRI-CIC Assay Working Group. A critical assessment for the value of markers to gate-out undesired events in HLA-peptide multimer staining protocols. J Transl Med. 2011;9:108. PubMed PMID: 21745365.
Janetzki S, Britten CM. The impact of harmonization on ELISPOT assay performance. Methods Mol Biol. 2012;792:25-36. PubMed PMID: 21956498.
van der Burg SH, Kalos M, Gouttefangeas C, Janetzki S, Ottensmeier C, Welters MJ, Romero P, Britten CM, Hoos A. Harmonization of immune biomarker assays for clinical studies. Sci Transl Med. 2011 Nov 9;3(108):108-144. PubMed PMID:22072636.